Abstract
Introduction: Ciltacabtagene autoleucel (cilta-cel), a chimeric antigen receptor T-cell (CAR-T) therapy with 2 B-cell maturation antigen (BCMA)-targeting single-domain antibodies, is being evaluated in patients (pts) with relapsed/refractory multiple myeloma (RRMM). In the phase 1b/2 CARTITUDE-1 (NCT03548207) study, a single dose of cilta-cel led to early, deep, and durable responses in heavily pretreated pts with MM, with a manageable safety profile (Berdeja, Lancet, 2021). At median 18 months of follow-up, the overall response rate (ORR) was 98%, with 80% of pts achieving stringent complete response. Median duration of response (DOR) was 21.8 months (95% CI, 21.8-not estimable); 18-month progression-free survival (PFS) and overall survival (OS) rates were 66% and 81%, respectively. Here, we report the efficacy and safety of cilta-cel in various subgroups of pts in CARTITUDE-1.
Methods: Eligible pts had MM and received ≥3 prior lines of therapy (LOT) or were double refractory to a proteasome inhibitor (PI) and immunomodulatory drug (IMiD), and had received a PI, IMiD, and anti-CD38 antibody. After apheresis, bridging therapy was permitted. Pts received a single cilta-cel infusion (target dose: 0.75×10 6 CAR+ viable T cells/kg; range 0.5-1.0×10 6) 5-7 days after lymphodepletion (300 mg/m 2 cyclophosphamide, 30 mg/m 2 fludarabine daily for 3 days). Primary objectives were to characterize cilta-cel safety, confirm the recommended phase 2 dose (phase 1b), and evaluate efficacy (phase 2). In this combined phase 1b and phase 2 analysis, cytokine release syndrome (CRS) graded by Lee et al (Blood 2014) criteria and neurotoxicity by CTCAE v5.0 were mapped to the ASTCT criteria for CRS and immune effector cell-associated neurotoxicity (ICANS), respectively. Efficacy and safety were evaluated in the following subgroups: ≥65 years of age, Black/African American, 3 prior LOT, ≥4 prior LOT, triple-class refractory, penta-drug refractory, standard- and high-risk cytogenetics, International Staging System stage III, bone marrow plasma cells at baseline (≤30%, >30 to <60%, and ≥60%), BCMA tumor expression at baseline (<80%, ≥80%), and presence of soft tissue plasmacytomas (bone-based and extramedullary).
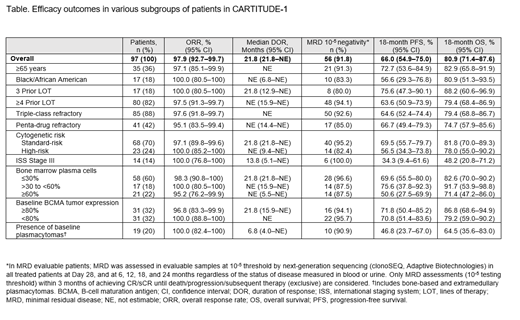
Results: As of February 11, 2021, 97 pts in the overall population received cilta-cel; 58.8% were male, median age was 61 years (range 43-78), and median time from diagnosis to enrollment was 5.9 years (range 1.6-18.2). ORR across all evaluated subgroups was comparable to the overall population, and was consistently high (range 95.1-100%) including in those with high-risk cytogenetics, ISS stage III MM, baseline bone marrow cells ≥60%, and baseline plasmacytomas (Table). Median DOR for most subgroups, including high-risk cytogenetics, was consistent with the overall population or not reached but was shorter in pts with ISS Stage III MM and baseline plasmacytomas (Table). Across all subgroups, 80%-100% of MRD-evaluable pts achieved MRD negativity (10 -5 threshold). 18-month PFS and OS rates were consistent with the overall population in most subgroups, including high-risk cytogenetics, and lower in ISS stage III and baseline plasmacytomas (Table). Incidence of CRS, ICANS, and other CAR T-cell neurotoxicities (events not reported as ICANS [ie, onset after a period of recovery from CRS and ICANS]) in various subgroups was consistent with the overall population, with no new safety signals.
Conclusions: At median follow-up of 18 months, a single infusion of cilta-cel yielded deep, durable responses in all evaluated subgroups in CARTITUDE-1. An ORR of 95%-100% was observed in pts across all subgroups, including those with high-risk cytogenetics, ISS stage III MM, baseline bone marrow cells ≥60%, and baseline plasmacytomas. In patients with ISS stage III and with baseline plasmacytomas, median DOR appeared shorter and 18-mo PFS and OS rates lower. Cilta-cel safety profile across the subgroups was consistent with the overall population, with no new safety signals. Ongoing evaluation of cilta-cel in the multicohort CARTITUDE-2 (NCT04133636) study in pts with unmet need in varying stages of treatment for MM will further explore the treatment benefit of cilta-cel, including in those with high-risk disease.
Jakubowiak: GSK: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Gracell: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Usmani: Janssen Oncology: Consultancy, Research Funding; Takeda: Consultancy, Research Funding, Speakers Bureau; SkylineDX: Consultancy, Research Funding; Sanofi: Consultancy, Research Funding, Speakers Bureau; Amgen: Consultancy, Research Funding, Speakers Bureau; Merck: Consultancy, Research Funding; Janssen: Consultancy, Research Funding, Speakers Bureau; Array BioPharma: Consultancy, Research Funding; GSK: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Celgene/BMS: Consultancy, Research Funding, Speakers Bureau; EdoPharma: Consultancy; Abbvie: Consultancy; Bristol-Myers Squibb: Research Funding. Berdeja: Bluebird bio, BMS, Celgene, CRISPR Therapeutics, Janssen, Kite Pharma, Legend Biotech, SecuraBio, Takeda: Consultancy; GSK, Ichnos Sciences, Incyte: Research Funding; EMD Sorono, Genentech: Research Funding; Celularity, CRISPR Therapeutics: Research Funding; Lilly, Novartis: Research Funding; Abbvie, Acetylon, Amgen: Research Funding; Poseida, Sanofi, Teva: Research Funding. Cohen: Oncopeptides: Consultancy; Genentech/Roche: Consultancy; Janssen: Consultancy; BMS/Celgene: Consultancy; Takeda: Consultancy; GlaxoSmithKline: Consultancy, Research Funding; AstraZeneca: Consultancy; Novartis: Research Funding. Hari: Karyopharm: Consultancy; Adaptive Biotech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Millenium: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Celgene-BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Schecter: Janssen: Current Employment, Current holder of stock options in a privately-held company. Yeh: Janssen: Current Employment. Olyslager: Janssen: Current Employment. Banerjee: Janssen: Current Employment, Current holder of individual stocks in a privately-held company. Jackson: Janssen: Current Employment; Memorial Sloan Kettering Cancer Center: Consultancy. Allred: Janssen: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Zudaire: Janssen: Current Employment. Deraedt: Janssen: Current Employment. Geng: Legend Biotech USA: Current Employment. Pacaud: Legend Biotech: Current Employment. Lin: Janssen: Consultancy, Research Funding; Vineti: Consultancy; Legend: Consultancy; Bluebird Bio: Consultancy, Research Funding; Novartis: Consultancy; Juno: Consultancy; Celgene: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Takeda: Research Funding; Sorrento: Consultancy; Merck: Research Funding; Gamida Cell: Consultancy. Martin: Janssen: Research Funding; GlaxoSmithKline: Consultancy; Oncopeptides: Consultancy; Amgen: Research Funding; Sanofi: Research Funding. Jagannath: Bristol Myers Squibb: Consultancy; Janssen Pharmaceuticals: Consultancy; Legend Biotech: Consultancy; Karyopharm Therapeutics: Consultancy; Takeda: Consultancy; Sanofi: Consultancy.
At the time of abstract submission, cilta-cel is being investigated for the treatment of multiple myeloma but is not yet approved.